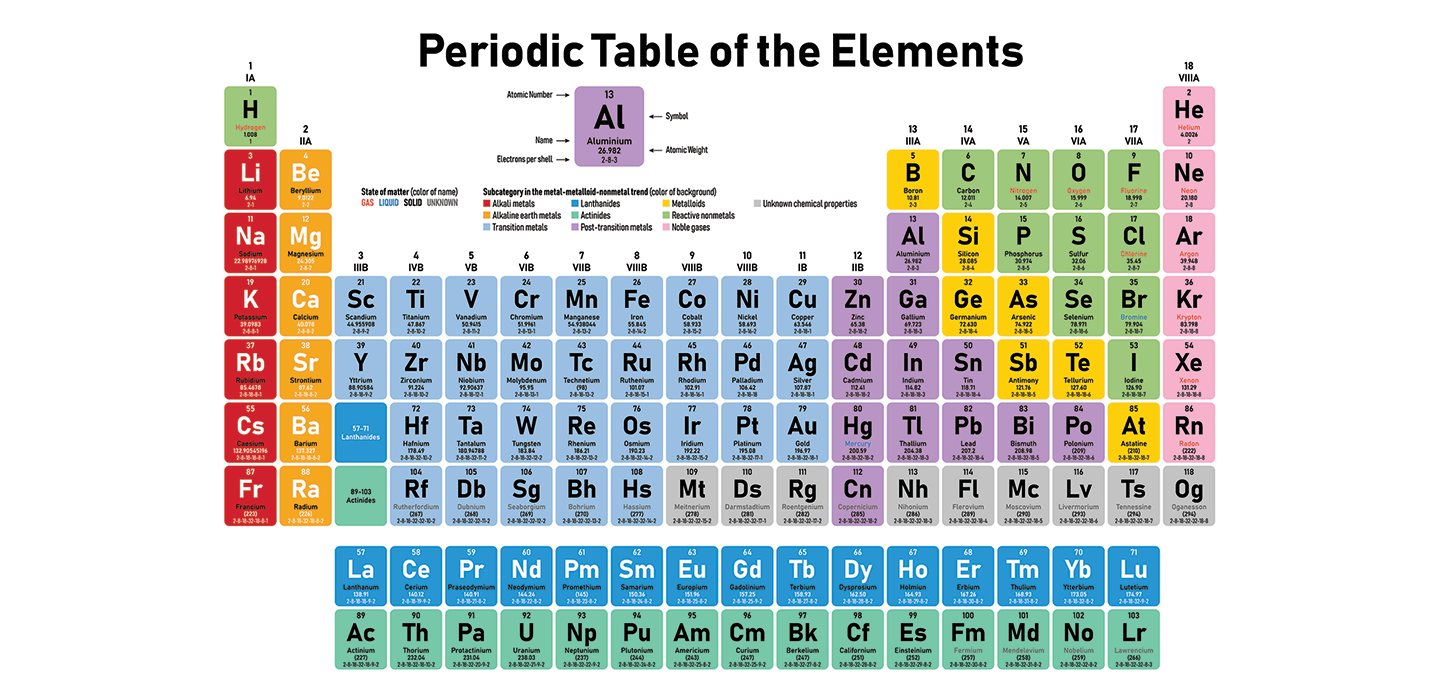
This is a chart that shows every one of the known chemical elements. The table is comprised of more than 100 squares. Each square addresses one element. A square contains a couple of letters that represent the element’s name, and numbers that tell about that element’s properties.
The area of each square in the table enlightens numerous things concerning every element. To start with, the elements are coordinated by atomic number, or the number of protons they that have. Those on top of the chart have the least protons. An element’s place additionally shows that it is so prone to respond. It additionally shows how its electrons are organized.
During the mid-1800s, numerous chemists searched for designs that made sense of how elements associated. In those days, researchers had barely any familiarity with the protons, neutrons and electrons that make up molecules. In any case, they comprehended that elements had different atomic loads. An atomic weight is the typical load of one particle of an element.
In 1869, the Russian physicist Dimitri Mendeleev arranged the 63 realized elements all together by their atomic loads. He saw patterns in the elements’ properties that shifted over unambiguous spans, or periods. Different researchers were chipping away at their own periodic tables, however Mendeleev distributed his table first.
The periodic table kept on developing as researchers found more elements. These incorporate the respectable gases, distinguished in 1890. This is a gathering of elements, for example, helium that could do without to respond with different elements. Beginning during the 1940s, researchers tracked down numerous new elements by impacting particles or bits of molecules.
Toward the finish of 2018, chemists affirmed four elements that had never been noticed. That carried the quantity of known elements to 118 and finished the seventh line of the table.
Reference: Carolyn Wilke @ snexplores